Progressus Investigationis in Polyurethanis Non-Isocyanatis
Ex quo anno 1937 introductae sunt, materiae polyurethane (PU) amplas applicationes in variis sectoribus invenerunt, inter quos transportatio, constructio, petrochemica, textilia, machinatio mechanica et electrica, industria aëronautica, cura valetudinis, et agricultura. Hae materiae in formis ut spumis plasticis, fibris, elastomeris, agentibus impermeabilibus, corio synthetico, tunicis, glutinis, materiis pavimenti et supplementis medicis adhibentur. PU traditionale imprimis ex duobus pluribusve isocyanatis una cum polyolis macromolecularibus et extensoribus catenae molecularis parvae synthesizatur. Attamen, toxicitas inherens isocyanatorum pericula significativa saluti humanae et ambitui imponit; praeterea typice ex phosgeno — praecursore valde toxico — et materiis crudis aminae correspondentibus derivantur.
Cum industria chemica hodierna rationes progressus viridis et sustinabilis persequatur, investigatores magis magisque in substitutione isocyanatorum cum materiis ecologicis intendunt, dum novas vias synthesis polyurethanorum non-isocyanaticorum (NIPU) explorant. Haec dissertatio vias praeparationis NIPU introducit, dum progressus in variis generibus NIPU recenset et eorum prospectus futuros discutit ut referentiam investigationibus ulterioribus praebeat.
1 Synthesis Polyurethanorum Non-Isocyanatorum
Prima synthesis compositorum carbamatis ponderis molecularis humilis utens carbonatis monocyclicis cum diaminis aliphaticis coniunctis peregre in annis 1950 facta est — momentum cardinale versus synthesim polyurethani non-isocyanaticam significans. Nunc duae methodologiae principales ad producendum NIPU exstant: prima reactiones additionis gradatim inter carbonatas cyclicos binarios et aminas binarias implicat; secunda reactiones polycondensationis implicat, intermedia diurethanica una cum diolis implicans, quae commutationes structurales intra carbamata faciliorem reddunt. Intermedia diamarboxylata per vias vel carbonatis cyclicis vel dimethylcarbonatis (DMC) obtineri possunt; fundamentaliter omnes methodi per greges acidi carbonici reagunt, functiones carbamati producentes.
Sequentes sectiones tres distinctas methodos ad synthesizandum polyurethanum sine usu isocyanatorum explicant.
1.1 Via Carbonatis Cyclica Binaria
NIPU synthesizari potest per additiones gradatim quae carbonatem cyclicum binarium cum amina binaria coniunctum implicant, ut in Figura 1 illustratur.
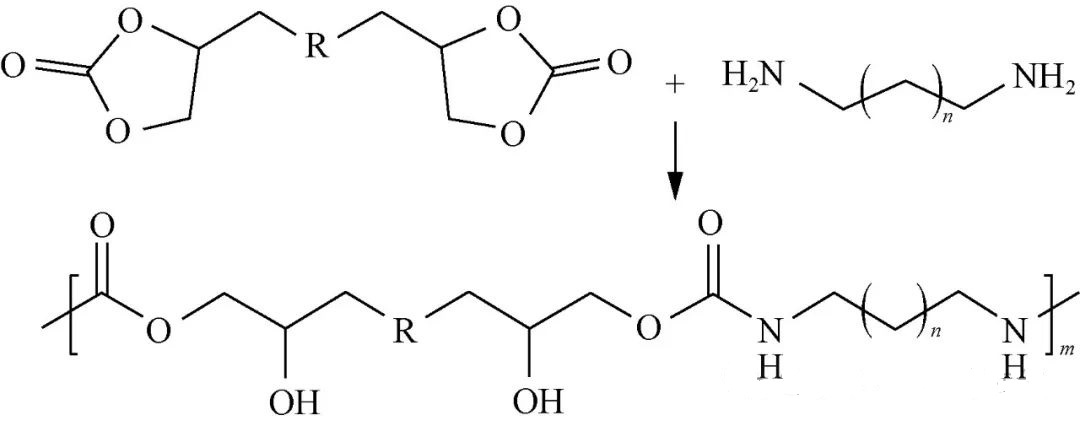
Ob multiplices greges hydroxylicos intra unitates repetitas secundum structuram catenae principalis praesentis, haec methodus plerumque producit quod polyβ-hydroxyl polyurethanum (PHU) appellatur. Leitsch et al., seriem polyetherum PHU excogitaverunt, polyetheres cyclico-carbonato terminatos una cum aminis binariis necnon moleculis parvis a carbonatis cyclicis binariis derivatis utentes — comparantes haec cum methodis traditis ad polyetherum PUs praeparandas adhibitis. Inventiones eorum indicaverunt greges hydroxylicos intra PHUs facile nexus hydrogenii cum atomis nitrogenii/oxygenii intra segmenta mollia/dura sitis formare; variationes inter segmenta mollia etiam mores nexuum hydrogenii necnon gradus separationis microphasium afficiunt, qui deinde proprietates functionis generales afficiunt.
Haec via, plerumque infra temperaturas excedentes 100°C peracta, nullos subproductos per reactiones generat, ita ut relative insensibilis ad humiditatem fiat, dum producta stabilia, sine volatilitate, producit; attamen solventia organica, polaritate forti insignita, ut dimethylsulfoxidum (DMSO), N,N-dimethylformamidum (DMF), etc., requiruntur. Praeterea, tempora reactionis extensa, ab uno usque ad quinque dies variantia, saepe pondera molecularia minora producunt, quae saepe sub liminibus circa 30kg/mol cadunt, productionem magnae scalae difficilem reddunt, plerumque propter sumptus altos, et insufficientem fortitudinem, quamvis PHUs resultantes exhibeant, quamvis applicationes promittant in campis materiarum amortiguantium, structurarum memoriae formae, formularum adhaesivarum, solutionum obductionis, spumarum, etc.
1.2 Via Carbonatis Monocylici
Carbonas monocylicus directe cum diamina reagit, dicarbamatum cum gregibus terminalibus hydroxylis habens producit, quod deinde interactiones speciales transesterificationis/polycondensationis una cum diolis subit, tandem NIPU structuraliter similem generans, quod visualiter per Figuram 2 depictum est.
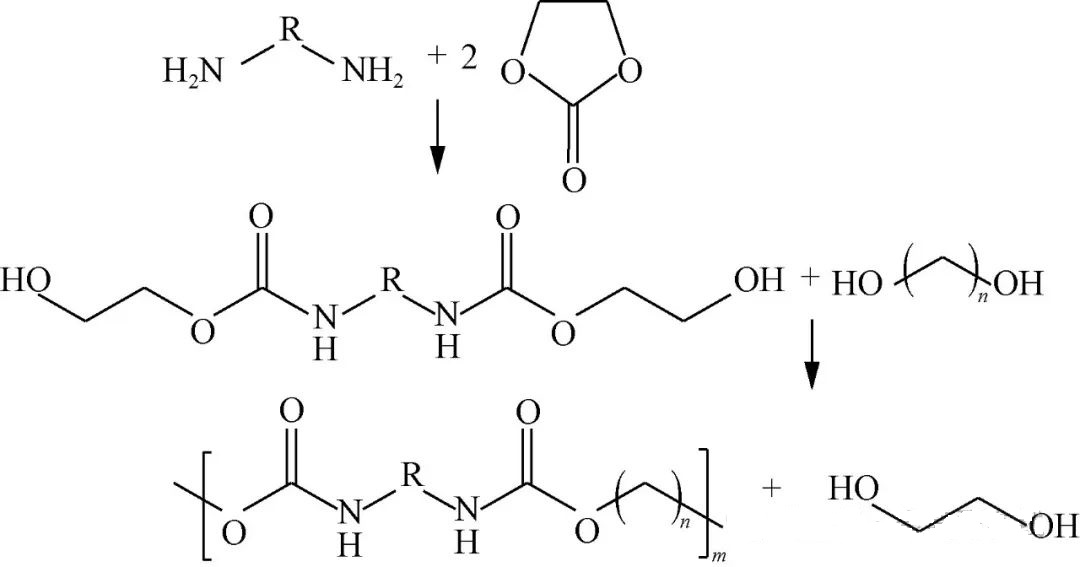
Inter varietates monocylicas vulgo adhibitas sunt substrata carbonata aethyleni et propyleni, ubi turma Zhao Jingbo apud Universitatem Technologiae Chemicae Pechinensem varias diaminas adhibuit, eas contra dictas entitates cyclicas reagendo, initio varios intermediarios dicarbamati structurales obtinens, antequam ad phases condensationis progrediens, polytetrahydrofuranediolo/polyether-diolis utens, formando prospere lineas productorum respectivas, proprietates thermicas/mechanicas impressas exhibentes, puncta liquefactionis circa 125~161°C extensa, robora tensile circa 24MPa attingentes, et elongationes fere 1476%. Wang et al. similiter combinationes DMC cum hexamethylenediamino/praecursoribus cyclocarbonatis pariter confectas adhibuerunt, derivata hydroxy-terminata synthetizantes, postea acida dibasica biobasata, ut acidis oxalicum/sebacicum/adipico-terephthalicis, subiecta, effectus finales ostendentes, robora tensile inter 13k~28k g/mol fluctuantia, elongationes inter 9~17 MPa variantes, et elongationes inter 35%~235%.
Esteres cyclocarbonici, sub condicionibus typicis, sine catalysatoribus necessariis efficaciter agunt, temperaturae inter 80°C et 120°C servantes; transesterificationes subsequentes plerumque systemata catalytica organostannica adhibent, processum optimum non excedens 200°C. Ultra meras conatus condensationis, quae input diolicum spectant, phaenomena autopolymerizationis/deglycolysis generationem facilitant, exitus desideratos methodologiam inherenter oecologicam reddit, praesertim residua methanolica/diolica parvarum moleculorum producens, ita alternativas industriales viabiles in futurum praebens.
Via 1.3 Dimethyli Carbonatis
DMC alternativam oecologice sanam/non toxicam praebet, numerosas partes functionales activas exhibens, inter quas configurationes methyl/methoxy/carbonyl, perfiles reactivitatis significanter augentes, quae initiales communicationes permittit, qua DMC directe cum diaminis interagat, intermediaria methyl-carbamate terminata minora formans, deinde actiones condensationis liquefactae incorporantes additionales constituentes parvarum catenarum extensorum diolicorum/maiorum polyolorum, quod tandem efficit ut structurae polymericae quaesitae, per Figuram 3 visualizatae, emergentiae ducunt.
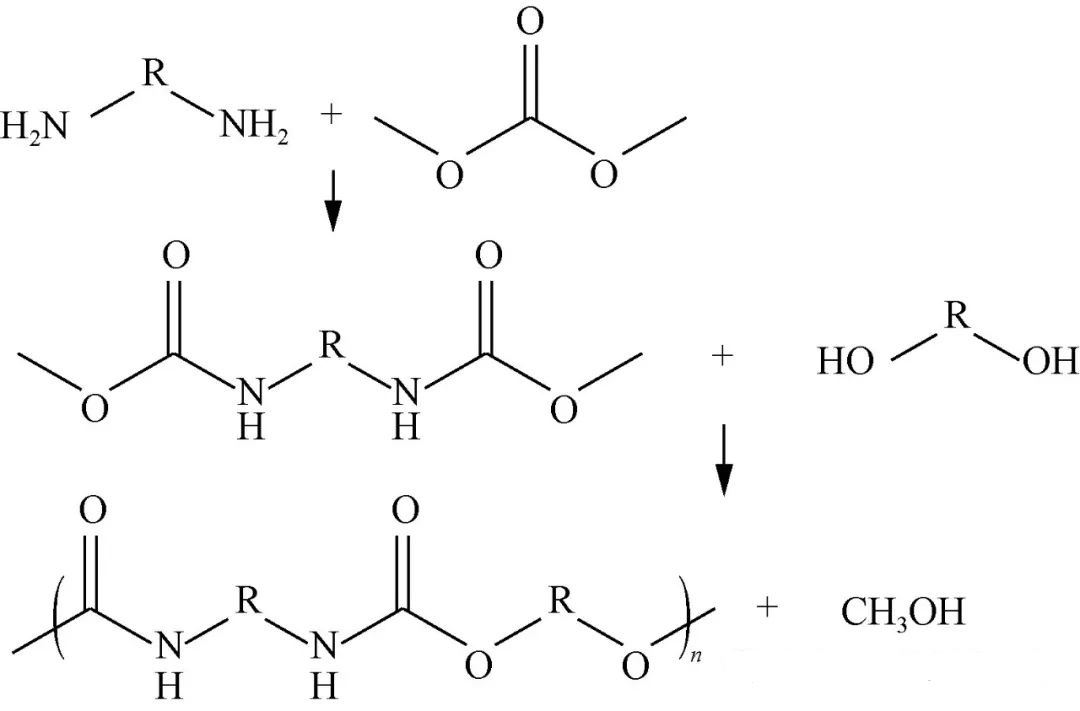
Deepa et al., dynamicis supra dictis usus, catalysi methoxidi natrii diversas formationes intermedias ordinans, deinde extensiones designatas involvens, compositiones segmentorum durorum aequivalentes in serie culminantes, pondera molecularia ad (3 ~ 20) × 10^3 g/mol appropinquantia, temperaturas transitionis vitreae (-30 ~ 120°C) pertingentibus. Pan Dongdong paria strategica, DMC hexamethylene-diaminopolycarbonate-polyalalcohols constantes, elegit, quae eventus notabiles praebent, cum metris roboris tensilis oscillantibus 10-15MPa et rationibus elongationis 1000%-1400% appropinquantibus. Investigationes circa varias influentias catenarum extendentiae praeferentias ostenderunt, quae selectiones butanedioli/hexanedioli favorabiliter alignant cum paritas numeri atomici aequalitatem servaret, promovens augmentationes crystallinitatis ordinatas per catenas observatas. Grex Sarazin composita ligninum/DMC una cum hexahydroxyamina integrantia paravit, proprietates mechanicas satisfacientes post processum ad 230℃ ostendens. Explorationes additionales ad derivandas non-isocyantas-polyureas, implicatione diazomonomerorum utentes, spectantes ad applicationes pigmentorum potentiales anticipandas, commoda comparativa prae contrapartibus vinyl-carbonicis emergentes, efficaciam sumptuum/vias acquisitionis latiores illustrantes. Diligentia debita de methodologiarum synthesis in massa plerumque ambitus temperaturae elevatae/vacui requirit, requisita solventium negans, ita flumina vastorum praesertim limitatorum ad methanolum/effluentia parvarum moleculorum-diolica minuens, paradigmata synthesis viridiora in universum constituens.
Duo segmenta mollia diversa polyurethani non-isocyanati
2.1 Polyether polyurethanum
Polyether polyurethanum (PEU) late adhibetur propter energiam cohaesionis vinculorum aethericorum humilem in unitatibus repetitis segmentorum mollium, rotationem facilem, flexibilitatem excellentem temperaturae humilis, et resistentiam hydrolysi.
Kebir et al. polyaetherem polyurethanum cum DMC, polyethylene glycol et butanediolo ut materiis primis synthetizaverunt, sed pondus moleculare parvum erat (7500 ~ 14800g/mol), Tg inferiorem quam 0℃, et punctum liquefactionis etiam humile (38 ~ 48℃), et robur aliique indicia difficulter necessitatibus usus satisfaciebant. Grex investigationis Zhao Jingbo ethylene carbonatum, 1,6-hexanediaminum et polyethylene glycol ad synthetizandum PEU adhibuit, quod pondus moleculare 31000g/mol, robur tensile 5 ~ 24MPa, et elongationem ad rupturam 0.9% ~ 1388% habet. Pondus moleculare seriei syntheticae polyurethanorum aromaticorum est 17 300 ~ 21 000g/mol, temperatura temperaturae (Tg) est -19 ~ 10℃, punctum liquefactionis est 102 ~ 110℃, robur tensile est 12 ~ 38MPa, et recuperatio elastica cum elongatione constanti 200% est 69% ~ 89%.
Grex investigationis Zheng Liuchun et Li Chuncheng intermedium 1,6-hexamethylenediaminum (BHC) cum dimethylcarbonato et 1,6-hexamethylenediamino paravit, necnon polycondensationem cum variis parvis moleculis, diolis catenae linearis et polytetrahydrofuranediolis (Mn=2000), perfecit. Series polyetheri polyurethanorum (NIPEU) via non-isocyanata praeparata est, et problema reticulationis intermediorum per reactionem solutum est. Structura et proprietates polyetheri polyurethani (HDIPU) traditionalis a NIPEU parati et 1,6-hexamethyleni diisocyanati comparatae sunt, ut in Tabula 1 demonstratur.
| Exemplum | Fractio massae segmenti duri/% | Pondus moleculare/(g·mol^(-1)) | Index distributionis ponderis molecularis | Robur tensile/MPa | Elongatio ad fracturam/% |
| NIPEU30 | 30 | 74000 | 1.9 | 12.5 | 1250 |
| NIPEU40 | 40 | 66000 | 2.2 | 8.0 | 550 |
| HDIPU30 | 30 | 46000 | 1.9 | 31.3 | 1440 |
| HDIPU40 | 40 | 54000 | 2.0 | 25.8 | 1360 |
Tabula 1
Resultata in Tabula 1 ostendunt differentias structurales inter NIPEU et HDIPU praecipue propter segmentum durum oriri. Grex ureae, ex reactione laterali NIPEU generatus, in catena moleculari segmenti duri temere inseritur, segmentum durum frangens ut vincula hydrogenii ordinata formet, unde nexus hydrogenii inter catenas moleculares segmenti duri debiles et crystallinitas segmenti duri humilis oriuntur, separationem phasium NIPEU humilem efficiens. Quam ob rem, proprietates mechanicae eius multo peiores sunt quam HDIPU.
2.2 Polyester Polyurethanum
Polyurethanum polyesteris (PETU), cum diolis polyesteris ut segmentis mollibus, bonam biodegradabilitatem, biocompatibilitatem, et proprietates mechanicas habet, et ad scaffolds textuum parandos adhiberi potest, quod est materia biomedica cum magnis occasionibus applicationis. Dioli polyesteris quae vulgo in segmentis mollibus adhibentur sunt polybutylenum adipatis diol, polyglycolum adipatis diol, et polycaprolactonum diol.
Antehac, Rokicki et al. ethyleni carbonas cum diamino et variis diolis (1,6-hexanediolo, 1,10-n-dodecanolo) reagebant ad varias NIPU obtinendas, sed NIPU synthesizatum minus pondus moleculare et Tg inferiorem habebat. Farhadian et al. polycyclicum carbonas utentes oleo seminis helianthi ut materia prima paraverunt, deinde cum polyaminibus biologicis mixtum, in lamina obductum, et ad 90 ℃ per 24 horas curatum ad pelliculam polyurethani polyesteris thermoindurentem obtinendam, quae bonam stabilitatem thermalem demonstravit. Grex investigationis Zhang Liqun ex Universitate Technologiae Sinarum Meridianarum seriem diaminorum et carbonatorum cyclicorum synthesizatam fecit, deinde cum acido dibasico biologico condensavit ad polyurethanum polyesteris biologicum obtinendum. Grex investigationis Zhu Jin apud Institutum Ningbo Investigationis Materiarum, Academiae Scientiarum Sinensis segmentum durum diaminodioli utentes hexadiamino et vinyl carbonato paravit, et deinde polycondensationem cum acido dibasico insaturato biologico fecit ad seriem polyurethani polyesteris obtinendam, quae ut pigmentum post curationem ultraviolaceam adhiberi potest [23]. Grex investigationis Zheng Liuchun et Li Chuncheng acidum adipicum et quattuor diola aliphatica (butanediol, hexadiol, octanediol et decanediol) cum diversis numeris atomicis carbonis adhibuit ad diola polyesterica correspondentia ut segmenta mollia praeparanda; grex polyurethani polyesterici non-isocyanati (PETU), nominati secundum numerum atomorum carbonis diolorum aliphaticorum, obtentus est per liquefactionem polycondensationis cum praepolymero segmenti duri hydroxy-signati, parato a BHC et diolis. Proprietates mechanicae PETU in Tabula II monstrantur.
| Exemplum | Robur tensile/MPa | Modulus elasticus/MPa | Elongatio ad fracturam/% |
| PETU4 | 6.9±1.0 | 36±8 | DCCLXXIII±35 |
| PETU6 | 10.1±1.0 | 55±4 | 568±32 |
| PETU8 | 9.0±0.8 | 47±4 | 551±25 |
| PETU10 | 8.8±0.1 | 52±5 | 137±23 |
Tabula II
Eventus ostendunt segmentum molle PETU4 maximam densitatem carbonylorum, fortissimum vinculum hydrogenii cum segmento duro, et infimum gradum separationis phasium habere. Crystallizatio tam segmenti mollis quam duri limitata est, ostendens punctum liquefactionis et vim tensilem humilem, sed maximam elongationem ad rupturam.
2.3 Polyurethanum polycarbonatum
Polyurethanum polycarbonatum (PCU), praesertim PCU aliphaticum, praeclaram resistentiam hydrolysi et oxidationi, bonam stabilitatem biologicam et biocompatibilitatem habet, et bonas opportunitates applicationis in campo biomedicinae praebet. In praesenti, pleraque NIPU praeparata polyaethylene polyola et polyester polyola ut segmenta mollia utuntur, et paucae relationes investigationum de polyurethano polycarbonato exstant.
Polyurethanum polycarbonatum non-isocyanaticum, a grege investigationis Tian Hengshui apud Universitatem Technologicam Sinarum Meridianarum paratum, pondus moleculare plus quam 50 000 g/mol habet. Influentia condicionum reactionis in pondus moleculare polymeri investigata est, sed proprietates eius mechanicae nondum relatae sunt. Grex investigationis Zheng Liuchun et Li Chuncheng PCU utens DMC, hexanediamino, hexadiolo et diolis polycarbonatis paravit, et PCU secundum fractionem massae unitatis repetitae segmenti duri nominavit. Proprietates mechanicae in Tabula 3 monstrantur.
| Exemplum | Robur tensile/MPa | Modulus elasticus/MPa | Elongatio ad fracturam/% |
| PCU18 | 17±1 | 36±8 | DCCLXV±24 |
| PCU33 | 19±1 | 107±9 | DCCLVI±33 |
| PCU46 | 21±1 | CL±16 | 407±23 |
| PCU57 | 22±2 | 210±17 | 262±27 |
| PCU67 | 27±2 | quadringenti±13 | 63±5 |
| PCU82 | 29±1 | 518±34 | 26±5 |
Tabula III
Eventus ostendunt PCU habere magnum pondus moleculare, usque ad 6×10⁴ ~ 9×10⁴ g/mol, punctum liquefactionis usque ad 137 ℃, et robur tensile usque ad 29 MPa. Hoc genus PCU adhiberi potest vel ut plastica rigida vel ut elastomer, quod bonam applicationem in agro biomedico habet (velut in structuris machinationis textuum humanorum vel materiis implantationis cardiovascularis).
2.4 Polyurethanum hybridum non-isocyanaticum
Polyurethanum hybridum non-isocyanaticum (NIPU hybridum) est introductio resinae epoxydicae, acrylati, silicae vel siloxani in structuram molecularem polyurethani ad retem interpenetrantem formandam, efficaciam polyurethani emendandam, vel polyurethano functiones diversas dandas.
Feng Yuelan et al. oleum soiae epoxy biologicum cum CO2 reagebant ad carbonatem pentamonicum cyclicum (CSBO) synthetizandum, et bisphenol A diglycidyl aetherem (resinam epoxy E51) cum segmentis catenae rigidioribus introduxerunt ad NIPU a CSBO cum amina solidificatum formatum ulterius emendandum. Catena molecularis segmentum catenae flexibile longum acidi oleici/acidi linoleici continet. Etiam segmenta catenae rigidiora continet, ita ut magnam firmitatem mechanicam et tenacitatem habeat. Quidam investigatores etiam tria genera praepolymerorum NIPU cum gregibus terminalibus furanorum per reactionem aperitionis celeris diethyleni glycolis bicyclici carbonatis et diamini synthetizaverunt, deinde cum polyestere insaturato reagebant ad polyurethanum molle cum functione auto-sanationis praeparandum, et feliciter magnam efficaciam auto-sanationis NIPU mollis assecuti sunt. NIPU hybridum non solum proprietates NIPU generalis habet, sed etiam meliorem adhaesionem, resistentiam corrosionis acidorum et alcali, resistentiam solventium et firmitatem mechanicam habere potest.
3 Prospectus
NIPU sine usu isocyanatis toxici praeparatur, et nunc in forma spumae, tegumenti, glutinis, elastomeri, aliorumque productorum investigatur, latamque applicationum possibilitatem habet. Pleraque tamen adhuc ad investigationes laboratorio circumscribuntur, nec productio magnae scalae existit. Praeterea, cum melioratione vitae hominum et continuo incremento postulationis, NIPU cum functione singulari vel multiplici magna directio investigationis facta est, ut puta antibacterialis, auto-reparabilis, memoria formae, ignifuga, alta resistentia caloris, et cetera. Quapropter, investigationes futurae comprehendere debent quomodo problemata principalia industrializationis perrumpant et directionem praeparationis NIPU functionalis explorare pergant.
Tempus publicationis: XXIX Augusti, MMXXIV


